Abstract
Background: According to the IPSS-R cytogenetic risk classification for myelodysplastic syndrome (MDS), patients with isolated deletion 5q (del5q), isolated deletion 20q (del20q), and del5q with an additional cytogenetic abnormality are classified as having good cytogenetics, similar to those with a normal karyotype. However the international database study, on which the IPSS-R cytogenetic risk classification is based, has its limitations in the small number of patients with del5q plus one additional cytogenetic abnormality (n=46). We sought to further define the prognostic significance of del5q with one additional cytogenetic abnormality in an evaluation of 86 patients.
Methods: We performed a retrospective analysis of 857 MDS patients with a karyotype of isolated del20q (n=160) or any karyotype involving del5q seen at a single tertiary cancer center between 1987-2021. Patients with del5q (n=697) were divided into 3 cohorts: isolated del5q (n=147), del5q with concomitant del20q (del5q+del20q, n=8), and del5q with a non-del20q cytogenetic abnormality (del5q+1, n=78). The remainder of del5q patients with complex karyotypes were excluded. Patient characteristics, laboratory values, and bone marrow data, including blast percentage and cytogenetics, were reviewed. Median OS was estimated using the Kaplan-Meier method and two-tailed log-rank test.
Results: The median age of patients was 70 years, with the majority being male (58%). Patients were more likely to present with lower risk disease (IPSS low to int-1, 78% of patients). The median follow-up was 2 years.
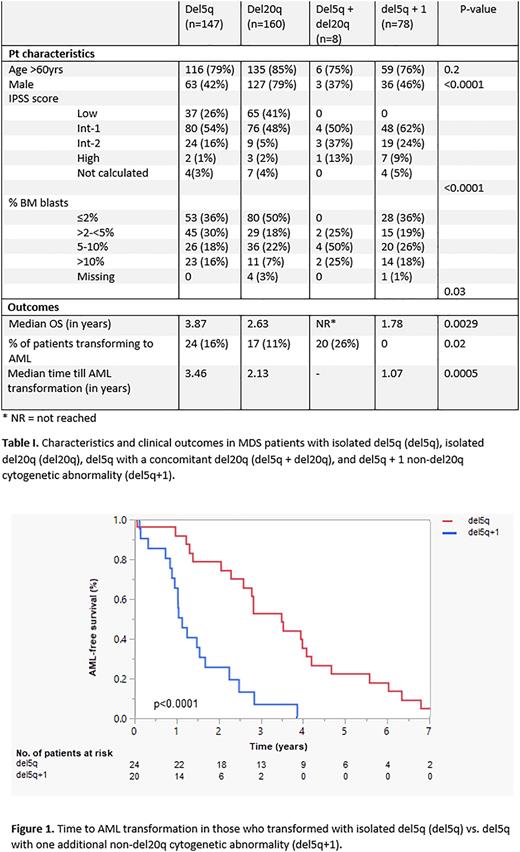
Patients with del5q+1 had an inferior survival when compared to patients with isolated del5q (median OS 1.78 years vs. 3.87 years, p=0.0047). Del5q+1 patients also had a higher risk of AML transformation (26% vs. 16%, p=0.098, table I) with a shorter median time to AML transformation (1.07 years vs. 3.46 years, p<0.0001, figure 1). A multivariate analysis of the combined del5q and del5q+1 cohort was performed, which evaluated age >60, percent blasts in the bone marrow, IPSS score, and cytogenetics. The only statistically significant prognostic factors for OS with a p-value <0.01 were age>60 (HR: 3.67 (2.24-6.0), p<0.0001 ) and cytogenetics (HR for del5q+1: 1.96 (1.3-2.9), p=0.001). The only prognostic factor for time to AML transformation with a p-value <0.01 was cytogenetic classification (HR for del5q+1: 5.2 (2.0-13.2), p=0.0006).
Patients with del20q had an OS between that of del5q (2.63 years vs. 3.87 years, p=0.14) and del5q+1 (2.63 years vs. 1.78 years, p=0.18). Del20q patients were less likely to transform to AML when compared to del5q+1 patients (11% vs. 26%, p=0.0035) with a trend towards longer time to AML transformation (2.1 years vs. 1.07 years, p=0.09). There were no statistically significant differences in AML transformation between the del5q and del20q cohorts (20% vs. 11%, p=0.1; median time to AML transformation: 3.46 years vs. 2.1 years, p=0.1).
The evaluation of OS and risk of AML transformation in patients with del5q and a contaminant del20q was limited due to low patient numbers (n=8, 1.1%). The median follow-up for these patients was 2.67 years, and none of the patients transformed to AML during this time. Two patients (25%) expired from the non-disease related causes of presumably underlying heart failure and massive pulmonary embolism following surgery. Interestingly, both deceased patients had >10% bone marrow blasts and were IPSS intermediate risk. The median OS for these 8 patients was not reached. However, the median follow-up time of 2.67 years may suggest their OS is better than those with del5q+1 (median OS ≥2.67 years vs. 1.78 years).
Conclusion: Contrary to the suggested IPSS-R cytogenetic risk stratification scheme, this analysis demonstrates a significant difference in terms of OS and risk of AML transformation when patients with isolated del5q or isolated del20q were compared to patients with del5q+1. Patients with del5q+1 had inferior median OS and a shorter time to AML transformation, suggesting more aggressive management may be warranted in this population. Though rare, patients with del5q+del20q may have better clinical outcomes than those with del5q+1, warranting further investigation into this group of patients.
Disclosures
Kadia:Pfizer: Research Funding; Ascentage: Research Funding; cellenkos: Research Funding; Servier: Consultancy; Delta-Fly: Research Funding; AstraZeneca: Research Funding; Amgen: Research Funding; cyclacel: Research Funding; Agios: Consultancy; Iterion: Research Funding; Regeneron: Research Funding; Astex: Honoraria; Glycomimetics: Research Funding; Genfleet: Research Funding; Astellas: Research Funding; Novartis: Consultancy; JAZZ: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; PinotBio: Consultancy; Abbvie: Consultancy, Research Funding. Jabbour:Spectrum: Research Funding; Bristol Myers Squibb: Other: Advisory Role, Research Funding; Genentech: Other: Advisory Role, Research Funding; Pfizer: Other: Advisory Role, Research Funding; Amgen: Other: Advisory Role, Research Funding; Adaptive Biotechnologies: Other: Advisory Role, Research Funding; AbbVie: Other: Advisory Role, Research Funding; Takeda: Other: Advisory Role, Research Funding. Pemmaraju:stemline: Consultancy; abbvie: Consultancy; immunogen: Consultancy; mustangbio: Research Funding; incyte: Consultancy; novartis: Research Funding; pacylex: Consultancy, Research Funding; samus: Research Funding; daiichi sankyo: Research Funding; cellectis: Research Funding; cellularity: Research Funding. Sasaki:Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Otsuka Pharmaceuticals: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees. Short:Stemline Therapeutics: Research Funding; Takeda Oncology: Consultancy, Research Funding; Astellas: Research Funding; Amgen: Consultancy, Honoraria; AstraZeneca: Consultancy; Pfizer: Consultancy; Novartis: Consultancy. Ravandi:Biomea Fusion, Inc.: Research Funding; Prelude: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Novartis: Consultancy; Syos: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; AstraZeneca: Consultancy; Astellas: Consultancy, Honoraria, Research Funding; Xencor: Research Funding. Alvarado:BerGenBio: Research Funding; Daiichi-Sankyo/Lilly: Research Funding; FibroGen: Research Funding; Sun Pharma: Research Funding; Astex Pharmaceuticals: Research Funding; Jazz Pharmaceuticals: Research Funding. Garcia-Manero:Acceleron Pharma: Consultancy; Genentech: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Curis: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Gilead Sciences: Research Funding; Novartis: Honoraria, Research Funding; Astex: Consultancy, Honoraria, Research Funding; Aprea: Honoraria. Kantarjian:NOVA Research: Honoraria; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz Pharmaceuticals: Research Funding; AbbVie: Honoraria, Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Research Funding; Amgen: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal